
Request a FREE SLActive Implant Trial Case
REGISTER BELOW
Brand new data on osseointegration potential
BENCHMARK PERFORMANCE PRE-CLINICAL STUDY¹
Results after 8 weeks1
Experimental replica
Roxolid® SLActive®
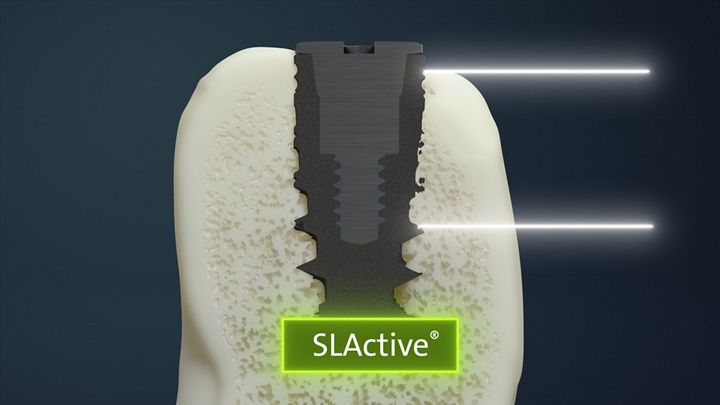
NobelActive®
TiUltra™
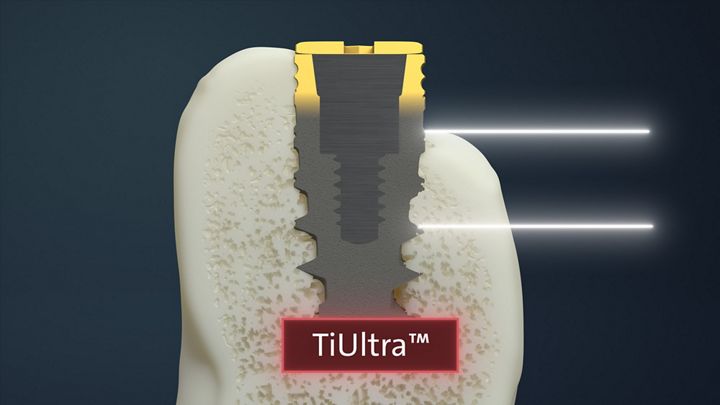
The Results
Brand new data
on osseointegration
* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001
The Study
Benchmark performance of anodized vs. sandblasted implant surfaces in an acute dehiscence type defect animal model
Objectives
Crestal bone formation represents a crucial aspect of the esthetic and biological success of dental implants. This controlled preclinical study analyzed the effect of implant surface and implant geometry on de novo crestal bone formation and osseointegration.
Materials and methods
Histological and histomorphometrical analysis was performed to compare three implant groups, i.e., (1) a novel, commercially available, gradient anodized implant, (2) a custom made geometric replica of implant “1”, displaying a superhydrophilic micro-rough large-grit sandblasted and acid-etched surface and (3) a commercially available implant, having the same surface as “2” but a different implant geometry. The study applied a standardized buccal acute-type dehiscence model in minipigs with observation periods of 2 and 8 weeks of healing.

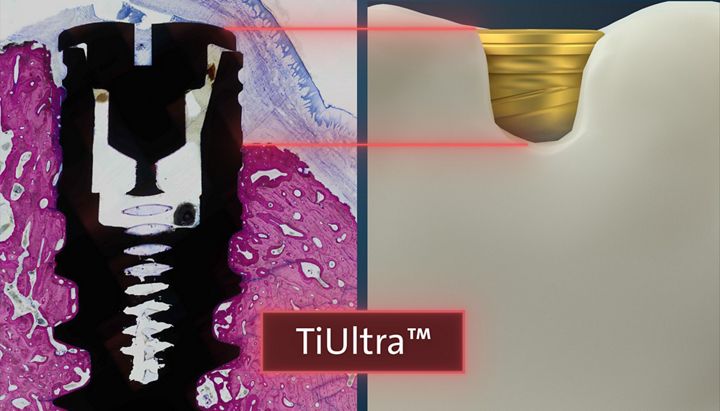
Results
The amount of newly formed crestal bone (BATA) around control group (2) and (3) was significantly increased when compared to the test group (1) at the 8-weeks healing time point. Similar results were obtained for all parameters related to osseointegration and direct bone apposition, to the implant surface (dBIC, VBC, and fBIC), demonstrating superior osseointegration of the moderately rough, compared to the gradient anodized functionalization. After 2 weeks, the osseointegration (nBIC) was found to be influenced by implant geometry with group (3) outperforming group (1) and (2) on this parameter. At 8 weeks, nBIC was significantly higher for group (2) and (3) compared to (1).
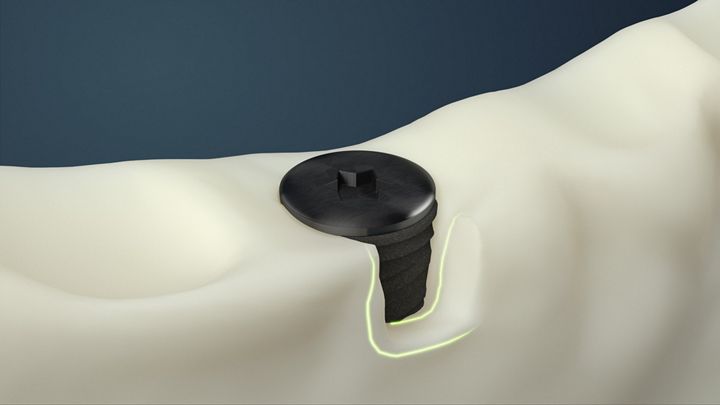
Acute dehiscence defect:
Roxolid® SLActive®
Bone healing after 8 weeks:
Roxolid® SLActive®

Acute dehiscence defect:
NobelActive® TiUltra™

Bone healing after 8 weeks:
NobelActive® TiUltra™

Conclusions
The extent (BATA) of de novo crestal bone formation in the acute-type dehiscence defects was primarily influenced by implant surface characteristics and their ability to promote osseointegration and direct bone apposition.
Osseointegration (nBIC) of the apical part was found to be influenced by a combination of surface characteristics and implant geometry. For early healing, implant geometry may have a more pronounced effect on facilitating osseointegration, relative to the specific surface characteristics.
Testimonial
Prof. Shahdad discusses the recently published pre-clinical study on implant surfaces
1 Shahdad S. et al. Benchmark performance of anodized vs. sandblasted implant surfaces in an acute dehiscence type defect animal model. Clin Oral Implants Res. 2022 Sep 19 2022.
TiUltra™ is a registered trademark of Nobel Biocare Services AG, Switzerland
