Mastering durability. High-density PTFE membrane
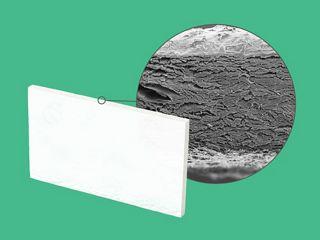
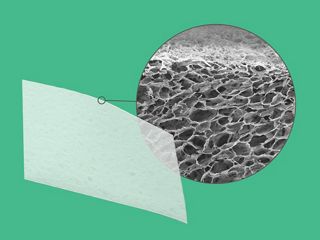
permamem® is an exceptionally thin, non-resorbable, biologically inert and biocompatible membrane made of high-density poly- tetrafluoroethylene (PTFE). permamem® maintains its structural characteristics both during the initial implantation and over time. Due to its dense structure the membrane acts as an efficient barrier against bacterial and cellular penetration, and may therefore be left in place for open healing in certain indications.
Features and benefits
permamem® is a 100% synthetic barrier membrane, thus any risk for disease transmission can be excluded.
The membrane is composed of biologically inert, high-density PTFE, which acts as an efficient barrier against bacterial and cellular penetration, and may therefore be used for open healing in socket and ridge preservation.
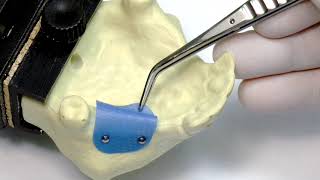
Easy handling thanks to its thin character (thickness ~ 0.08 mm). In open healing procedures, permamem® may easily be removed after the desired healing time with a pair of tweezers. The rounded edges of the membrane avoid traumatization of the soft tissue.
Watch and learn
permamem® use cases on youTooth

DO YOU HAVE MORE QUESTIONS ABOUT PERMAMEM®?
Download Straumann® Regenerative Solutions brochure to learn more about permamem® or share your questions with us.
Looking for additional information? You'll find them in the Download Center.