Introduction
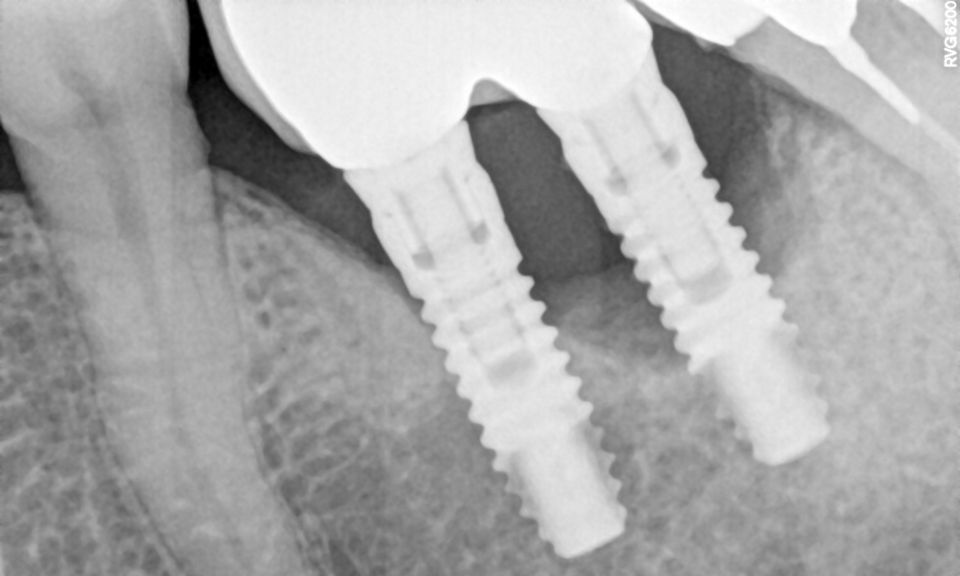
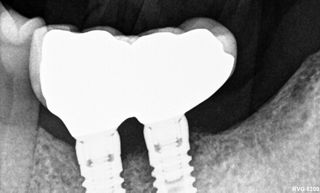
Peri-implant diseases are inflammatory conditions that affect the soft and hard tissues surrounding dental implants. These conditions are categorized primarily into two types: peri-implant mucositis and peri-implantitis. Peri-implant mucositis is characterized by inflammation confined to the peri-implant soft tissues without bone loss. This condition is considered reversible with appropriate intervention. In contrast, peri-implantitis extends beyond the soft tissues, involving the progressive loss of supporting alveolar bone, which can severely compromise the structural integrity of the dental implant. Peri-implantitis therefore represents a significant threat to the long-term success and survival of dental implants and, if not managed effectively, can ultimately lead to implant failure and loss.1,2
The pathogenesis of peri-implantitis is closely linked to the formation and maturation of a bacterial biofilm on the implant surface. This biofilm, a complex and resilient community of microorganisms encased in an extracellular polymeric matrix, is a critical etiological factor in the onset and progression of peri-implant diseases.3 The host immune response to these biofilms is characterized by the stimulation of inflammatory cells, including neutrophils and macrophages, which release pro-inflammatory cytokines and enzymes that degrade bone tissue. This inflammatory cascade leads to peri-implant bone resorption, jeopardizing the stability of the implant.4 Given the central role of biofilm in disease progression, effective biofilm removal is key in the management of peri-implantitis.
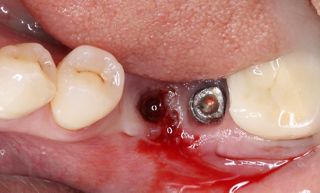
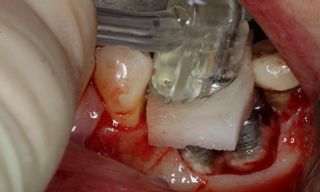
Treatment strategies for peri-implant diseases have evolved to include various approaches, often requiring a combination of mechanical debridement, antimicrobial therapy, and, in more advanced cases, surgical intervention. Mechanical debridement remains a cornerstone of treatment, focusing on the physical removal of biofilm and calculus from the implant surface. This may involve the use of specialized instruments such as ultrasonic scalers, titanium curettes, or air-polishing devices. In recent years, electrolytic cleaning has emerged as a novel approach for biofilm removal, utilizing low-level electrical currents to disrupt and detach bacterial biofilms from the implant surface.
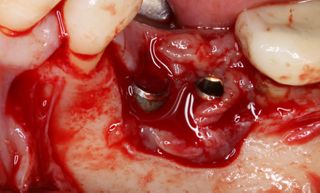
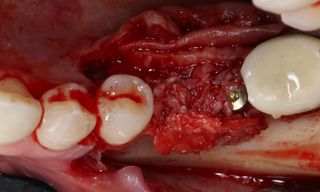
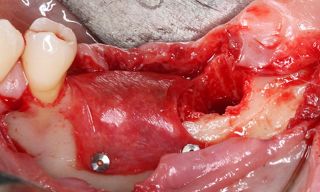
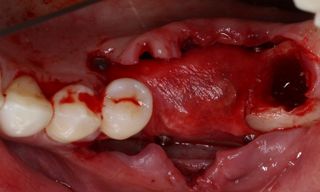
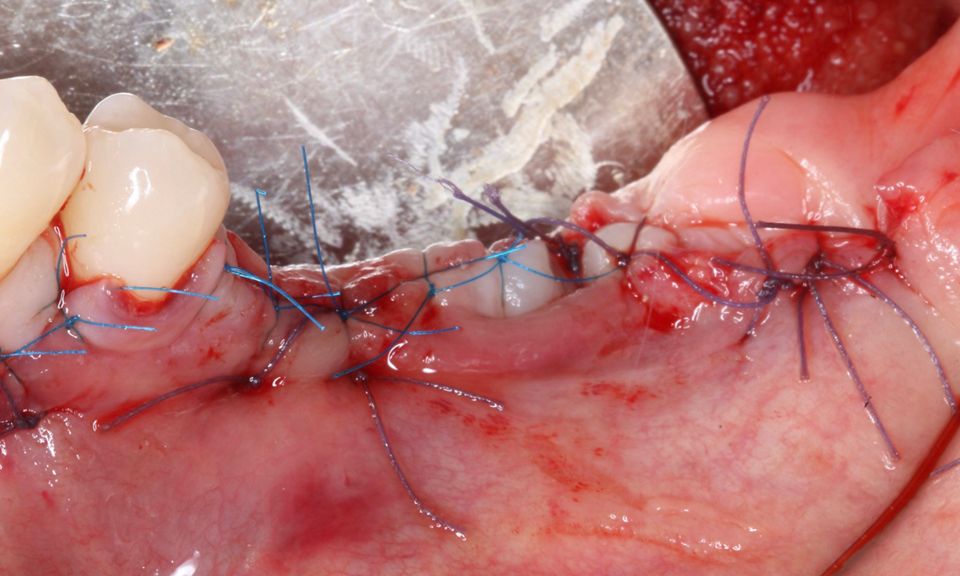
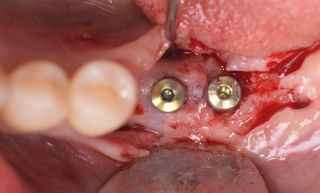
However, in cases of established peri-implantitis, non-surgical approaches may be insufficient to fully resolve the disease, and surgical interventions are often necessary to achieve thorough decontamination of the implant surface and to facilitate the regeneration of lost peri-implant tissues. The goal of these procedures is to re-establish a stable and healthy peri-implant environment conducive to the long-term stability of the implant. 5,6
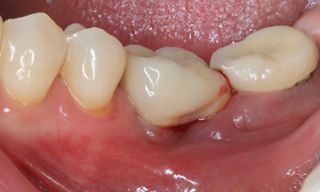
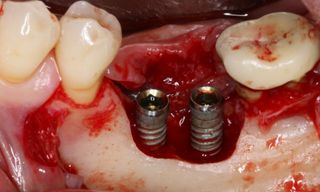
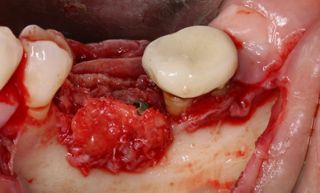
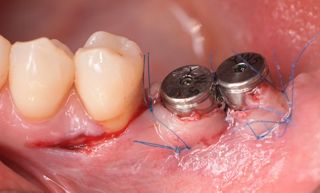
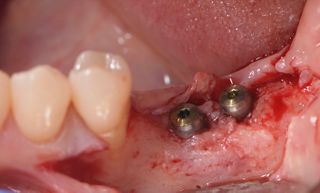
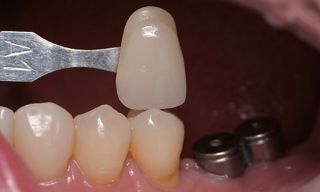
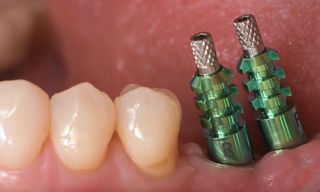
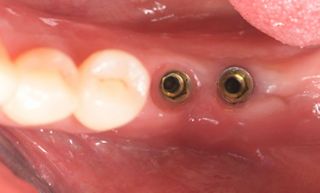
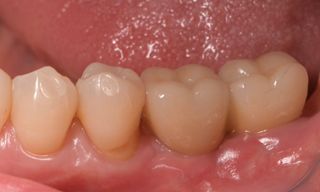
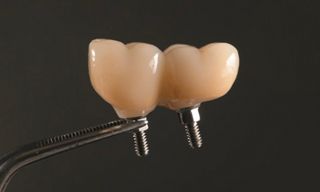
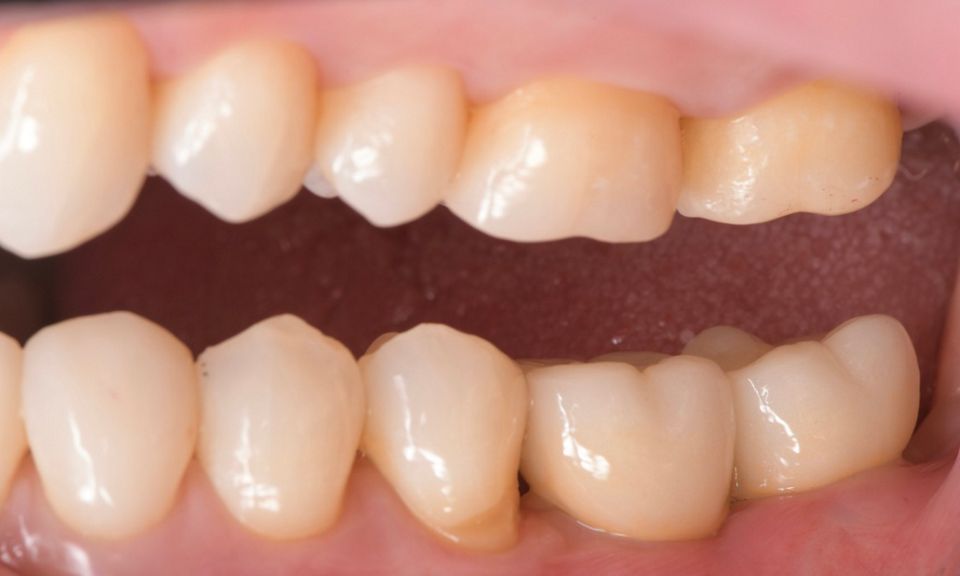
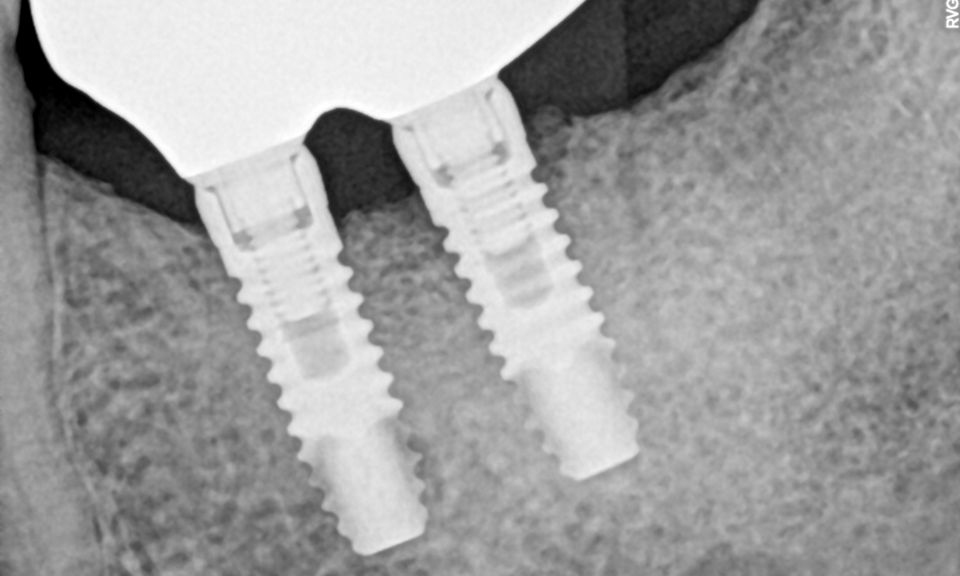
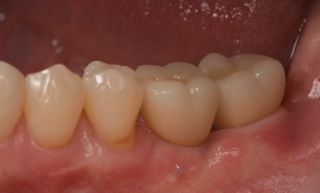
In this clinical case, we describe the successful management of peri-implantitis through a comprehensive treatment that integrates meticulous mechanical cleaning with regenerative techniques. This case highlights the critical importance of the efficacy of biofilm removal strategies and the application of regenerative procedures to achieve optimal clinical outcomes. The integration of these techniques not only stops the progression of the disease, but also facilitates the regeneration of lost bone and soft tissue, thereby ensuring the long-term stability and function of the dental implant.