The specialist society founded in Switzerland in 2018 is comprised of a Europe-wide network of scientifically renowned experts from clinical practice, universities and quality-oriented suppliers in the field of dental and ceramic implantology. In October 2018 the first joint official statement from science and industry was issued, outlining the current status of dental implantology with ceramic implants. Dr. Röhling, was there a specific occasion that marked the statement on two-piece ceramic implants issued some three years later?
Röhling: Essentially, one of the stated aims of the ESCI is to spark up conversations about topics and to develop concepts with which it can support its members in their work with ceramic implants in daily practice. The first official statement from the science and industry that you mentioned is considered a particular success of the first ESCI council, where specialists with a great deal of expertise in ceramic implantology held a round table discussion with representatives of the leading manufacturers. Our ESCI members very specifically looked at two-piece treatment concepts with ceramic implants with an aim to develop an argumentation that can also be used with the private health insurances when it comes to their obligation to reimburse costs.
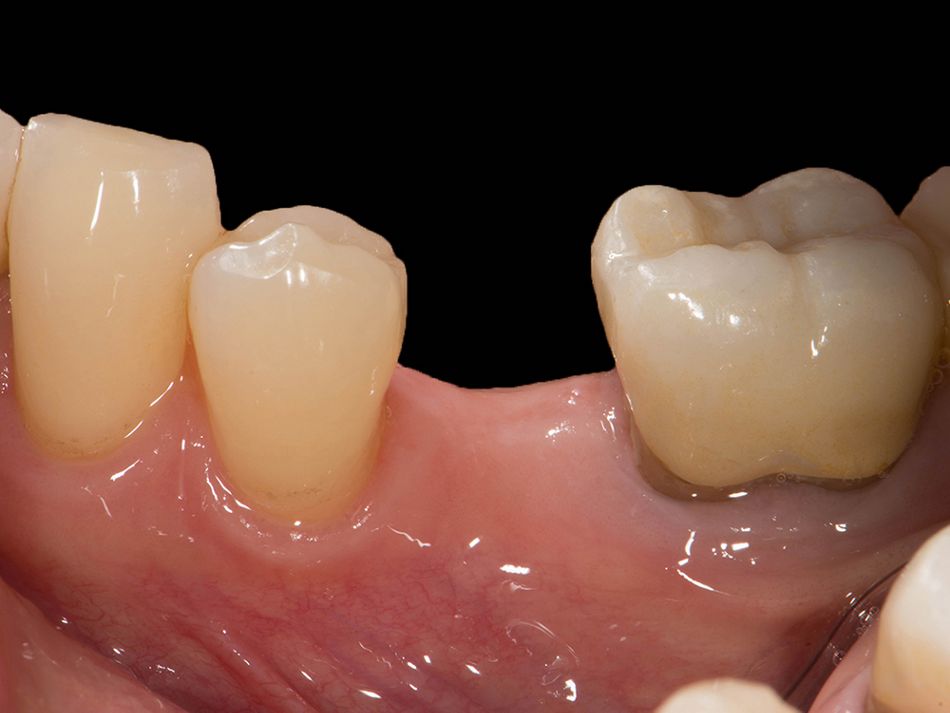
We already have the scientific data; so now we need to take an evidence-based approach to discuss and address questions that remain open. We have found that treatment with one-piece ceramic implants has been agreed and the costs covered, but that treatment with two-piece ceramic implants is again and again rejected on the grounds that the data are too sparse.
What is the current data status for two-piece zirconia implants? To what extent can the argument brought forward by the insurance companies, that there is not enough data, be disproved?
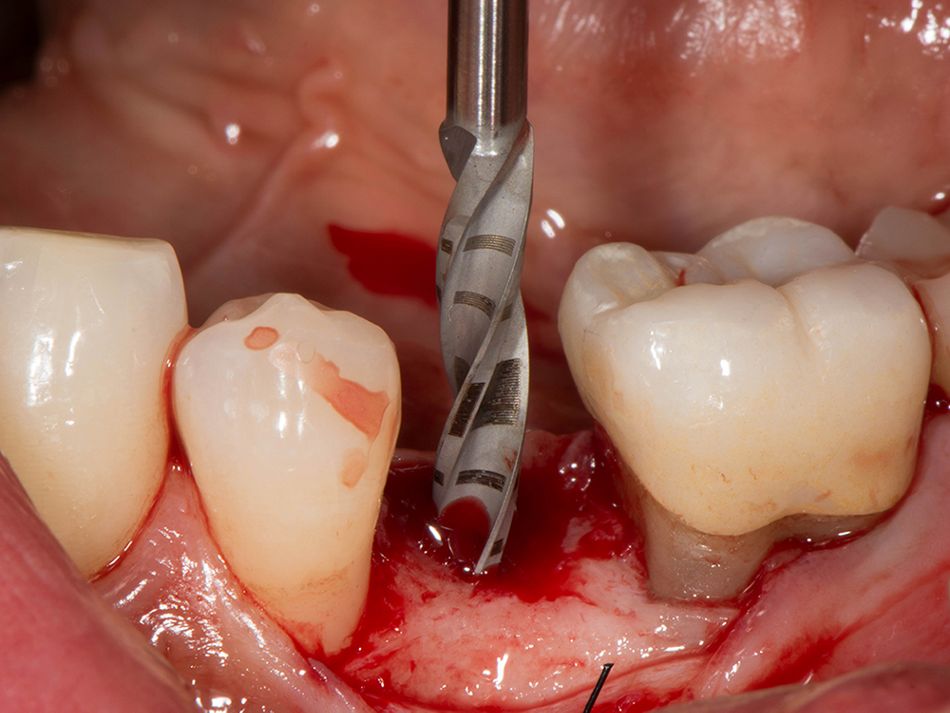
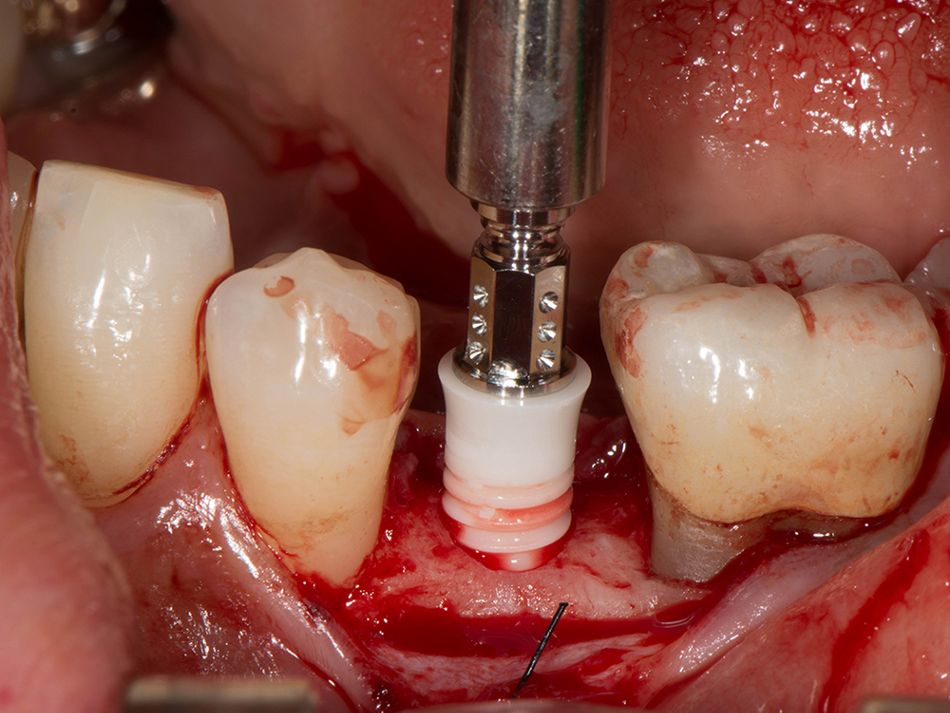
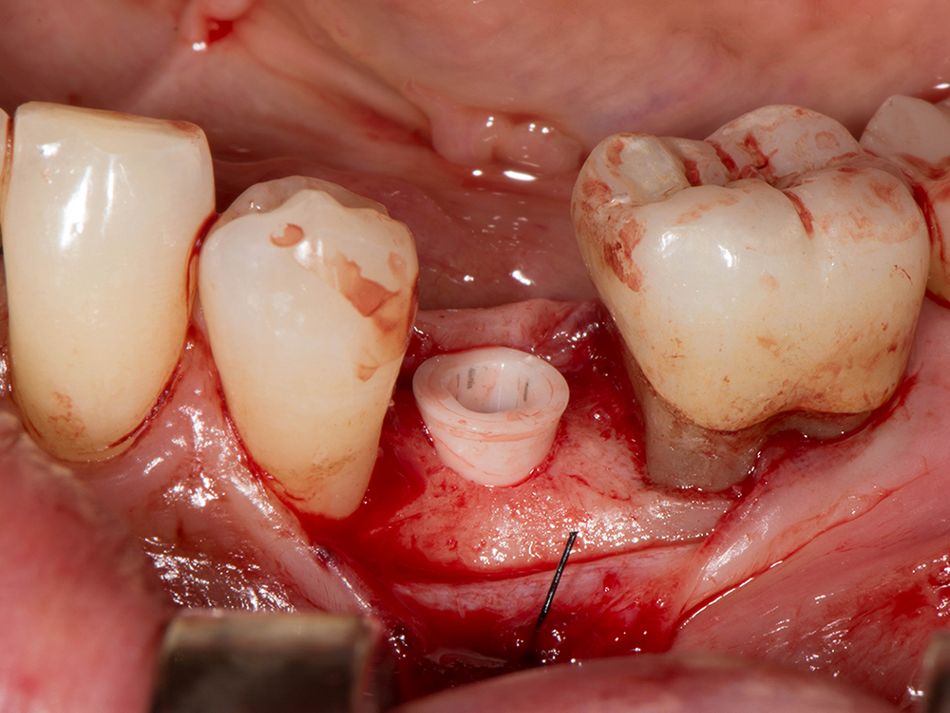
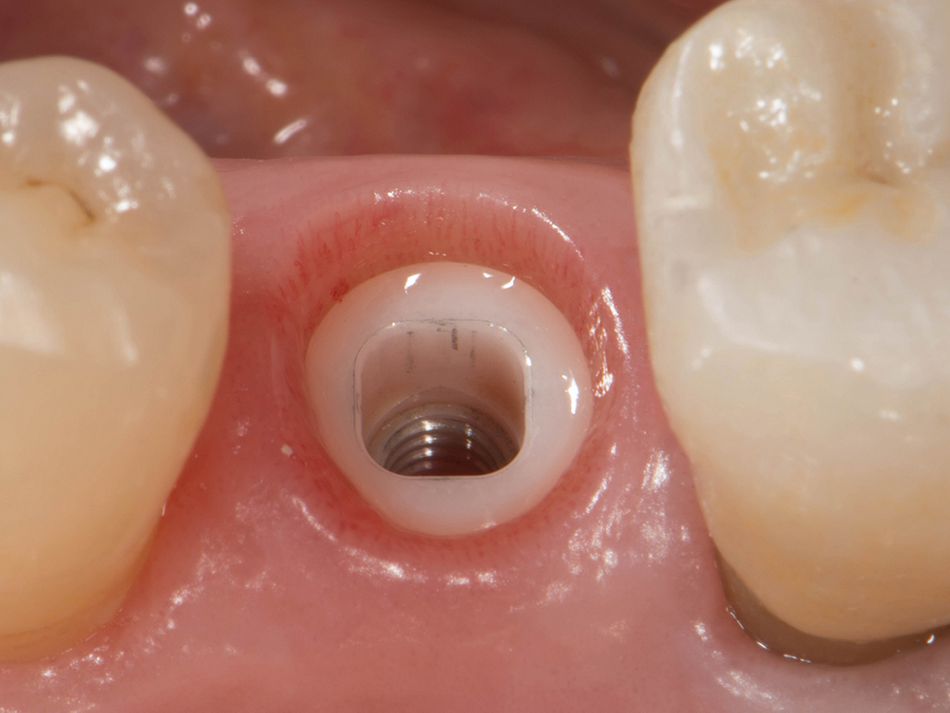
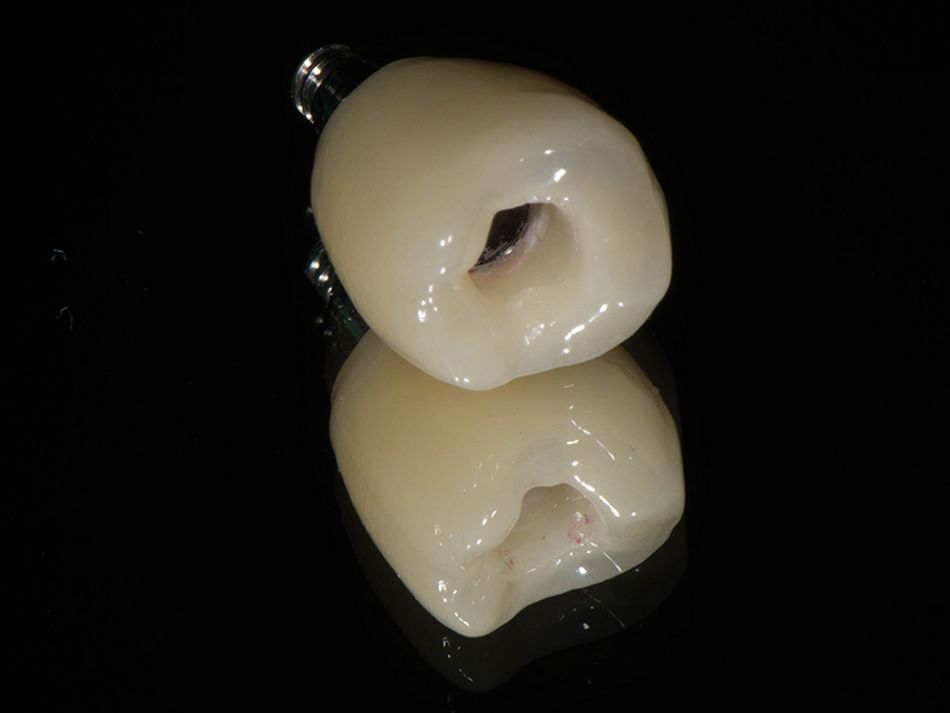
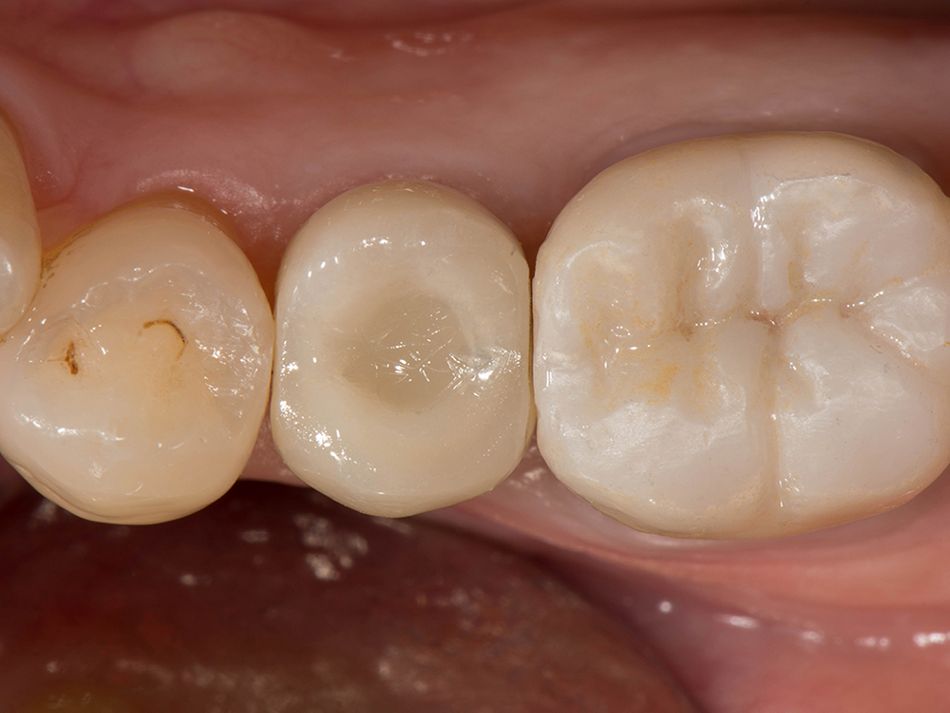
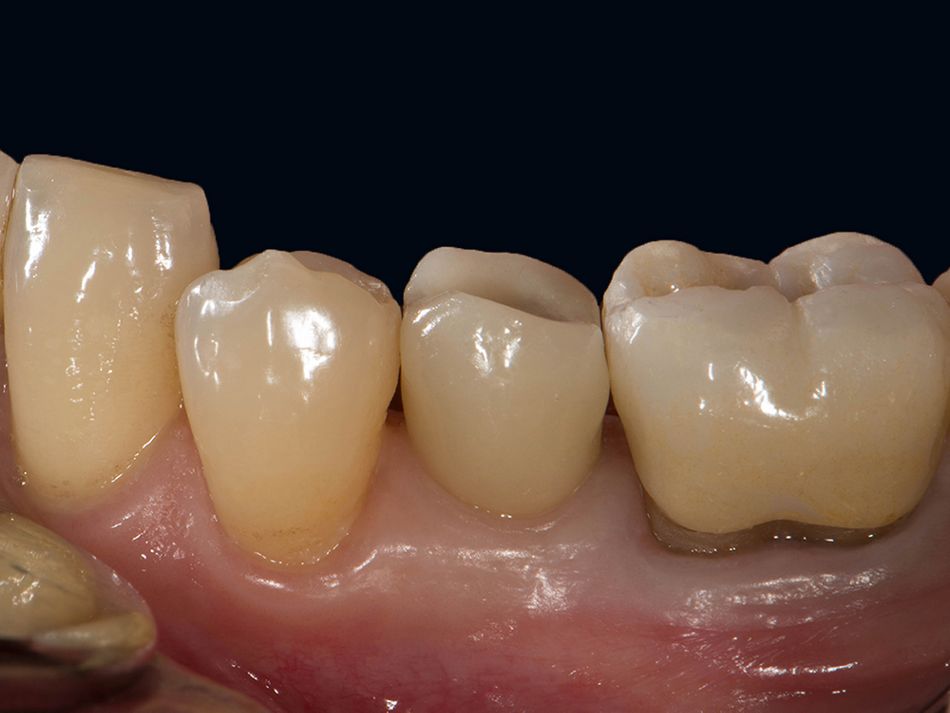
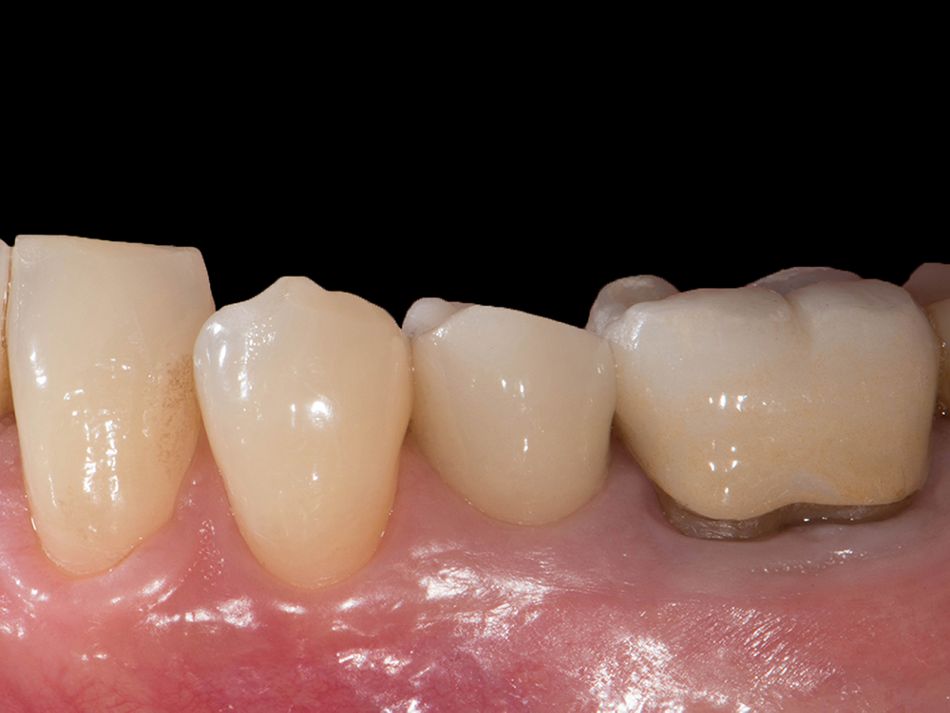
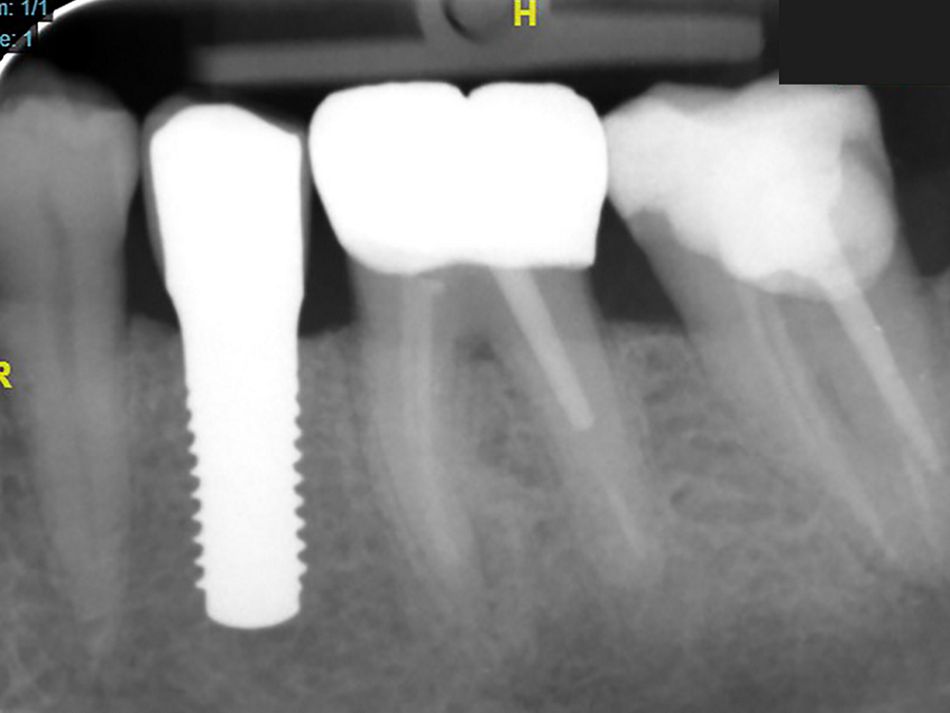
Röhling: Even though a significant majority of data published to date reference one-piece implant systems from high-performance zirconia, and data on zirconia implants with a two-piece implant design are currently limited, the following is apparent: A meta-analysis performed by our study group into which clinical studies have been integrated has shown that there is no difference in terms of survival rates between one-piece and two-piece implant designs. Even though meta-analyses to estimate the survival rates currently only include 1 to 2 years of data, individual studies report on longer clinical follow-up periods. There are currently clinical data available for commercially available zirconia implants covering follow-up periods of up to five years that show functional loading with 95% survival rates. There are enough data, and we also believe there is enough experience, to be able to recommend two-piece components with zirconia implants. The surfaces have been very thoroughly examined and are the same for one-piece and two-piece implants. So, in other words, one-piece and two-piece zirconia implants have the same degree of osseointegration and biological integrity. Two-piece ceramic implant connections from leading manufacturers have since been scientifically tested and classified as suitable for clinical application, and so it can be concluded that two-piece treatment concepts are just as suitable for practical use, while experts agree that the two-piece zirconia implant concept is suitable for clinical use after properly assessing the indication and explaining the procedure to the patient. What is problematic here, however, is that the industry has different production standards and quality control standards, which then directly affect the reliability of the ceramic implants and prosthetic connection.
The ESCI statement is attached to the relevant literature sites...