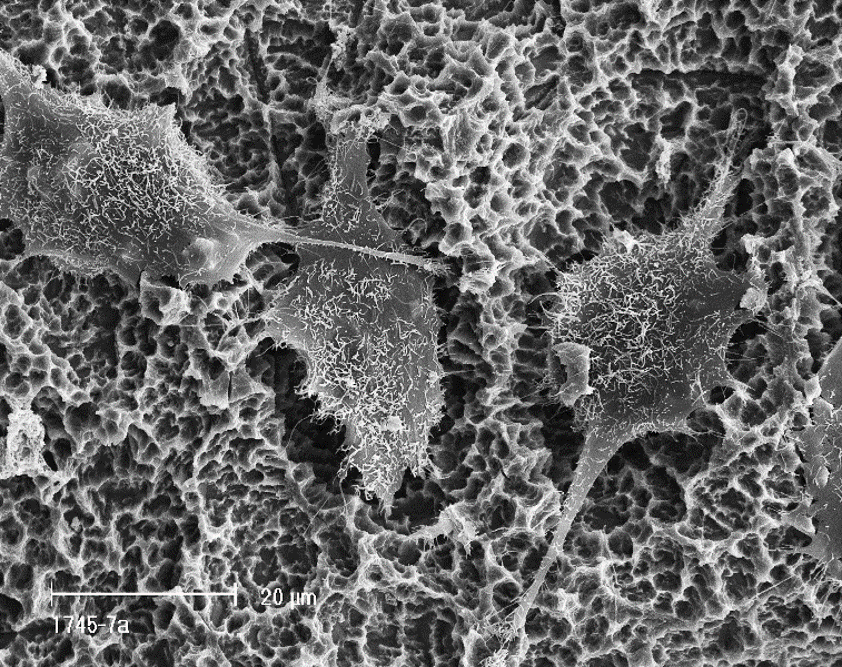
Understanding osseointegration
Our comprehension of the processes governing osseointegration has experienced a remarkable evolution1. Initially, osseointegration was primarily interpreted as unimpeded bone formation around bioinert materials1–3. In the 1990s, cell and molecular biology research began focusing on surface-adherent osteogenic cells and later also osteoclasts, which allowed describing osseointegration and peri-implant bone apposition as the net sum of bone forming and resorbing processes4. Nowadays, researchers started describing osseointegration in a more comprehensive way5,6. The immune system, a previously frequently overlooked factor, is currently increasingly recognized to play a pivotal role in regulating and mediating the processes governing both short- and long-term integration of implants7,8. This novel osteoimmunological description of osseointegration has also revealed the importance of Macrophages9,10. Of significant importance was the finding that Macrophages, depending on their environment can shift their status from a secretory pro-inflammatory “M1” into a re-generative “M2” phenotype. The phenomenon of Macrophage polarization is now being considered a key determinant for the type, magnitude, and duration of the inflammatory response to an implanted material9–11. In conjunction with other processes, it determines whether an inflammatory response may resolve into a healing and osseointegration pattern or persist and trigger processes like fibrous encapsulation, bone resorption, and ultimately osseointegration failure12. Inflammatory processes can have a dual role in osseointegration. On the one hand, transient inflammatory reactions are vital in promoting bone formation and implant integration. Persistent inflammation is, however, closely connected to a bone resorptive pattern, which can ultimately negatively influence the long-term survival and success of osseointegrated implants12–14. Components of both the innate and adaptive immune systems have been found to influence peri-implant bone formation and loss. This finding supports the notion that the inflammatory status and response around an implant is dynamic, complex, and patient-specific8,13. The implant material and its putative migration into peri-implant tissues has recently received increasing attention as a possible nonplaque-related inflammatory co-stimulus for peri-implant bone loss8,13,15–18. While a unidirectional causative relation between this process and peri-implant bone loss remains controversial, the aspect itself supports the importance of implant materials13,19.