Introduction
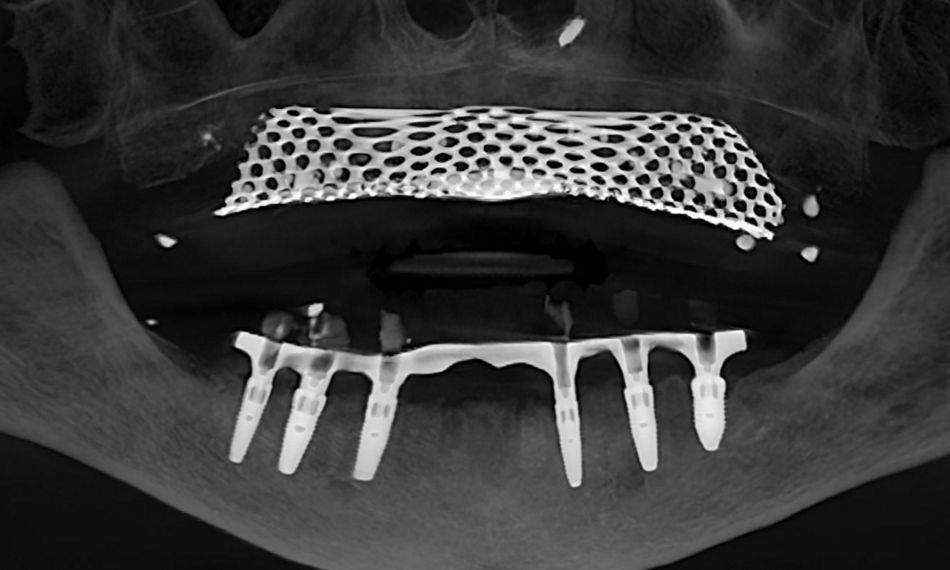
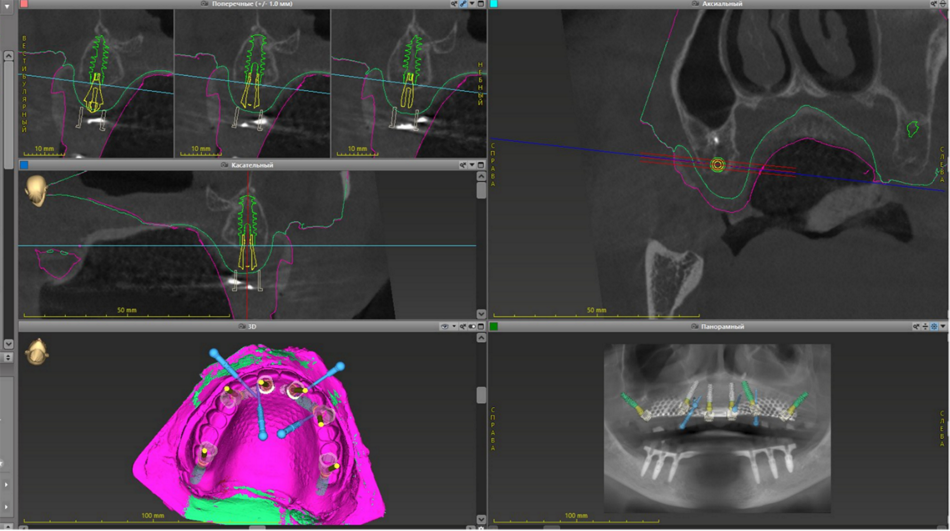
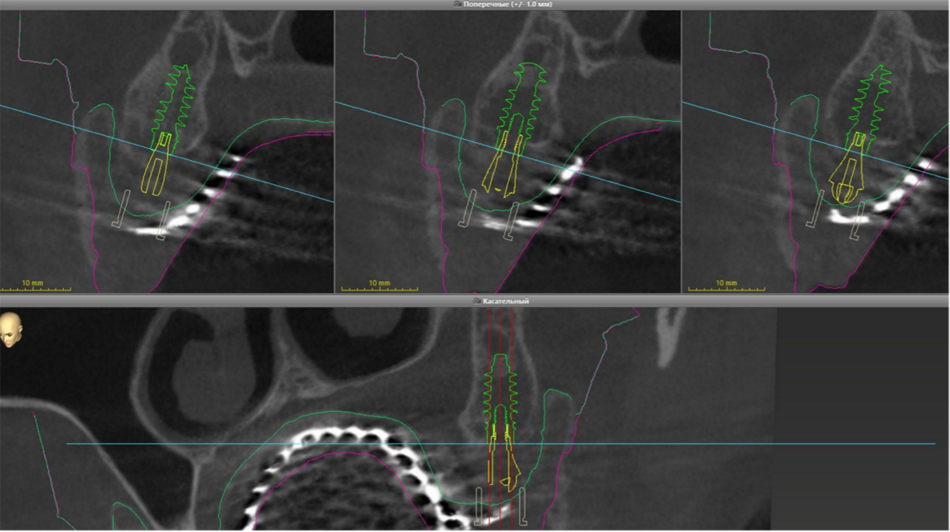
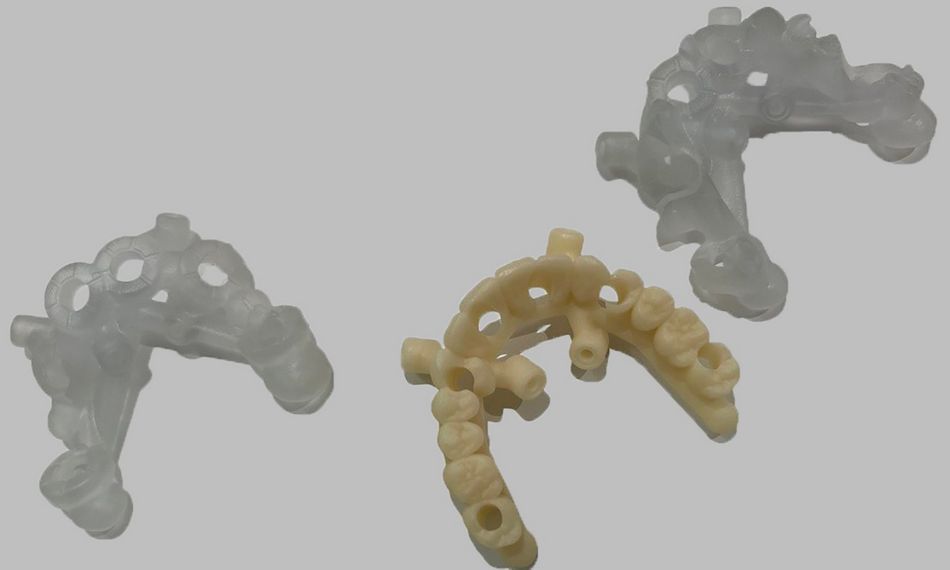
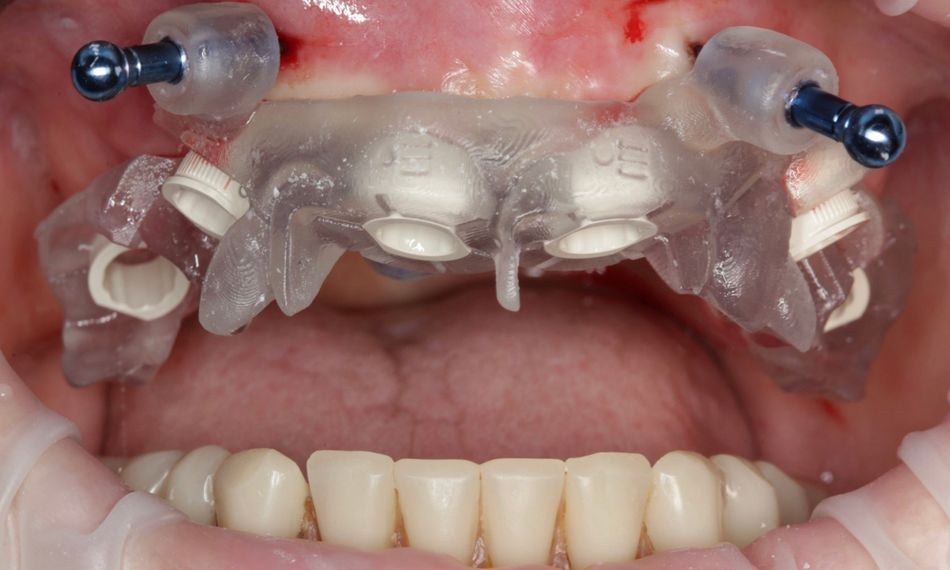
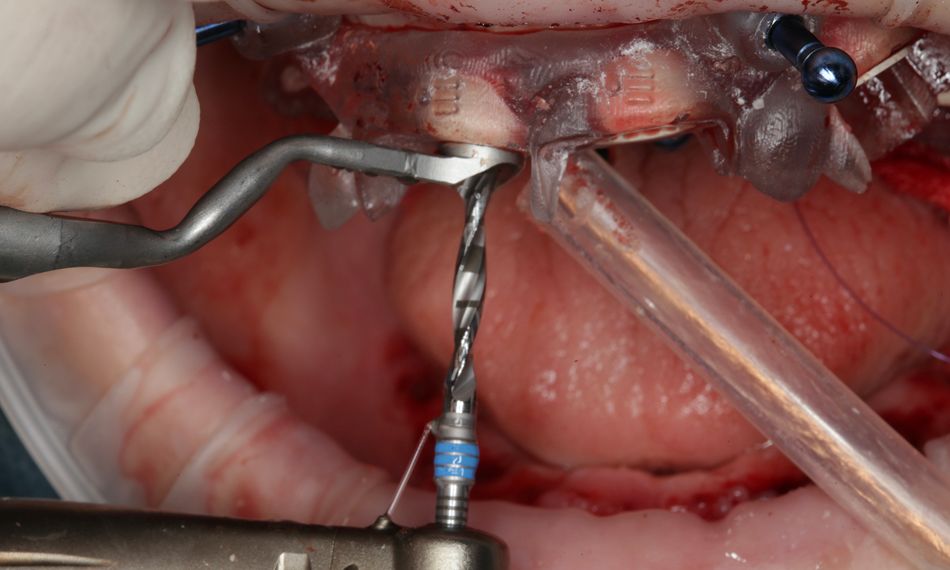
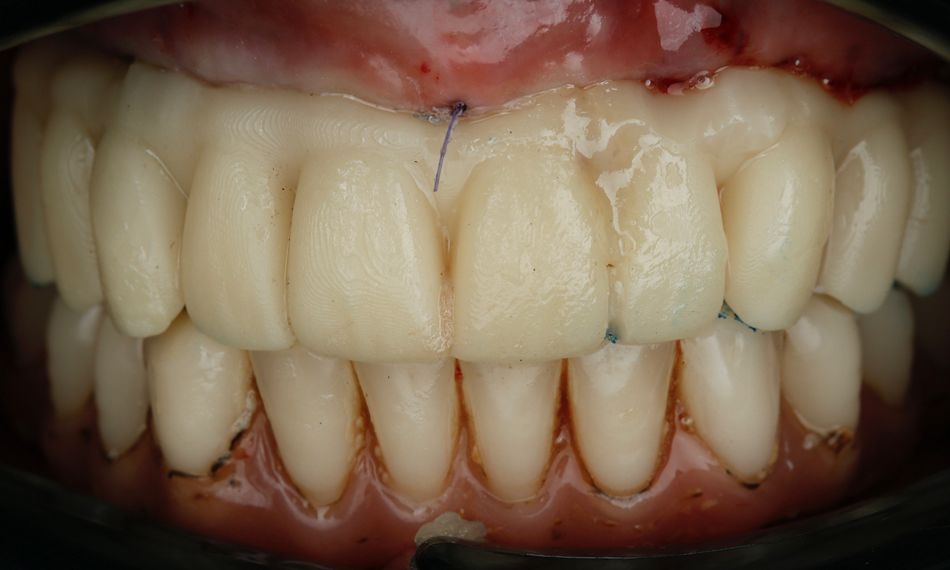
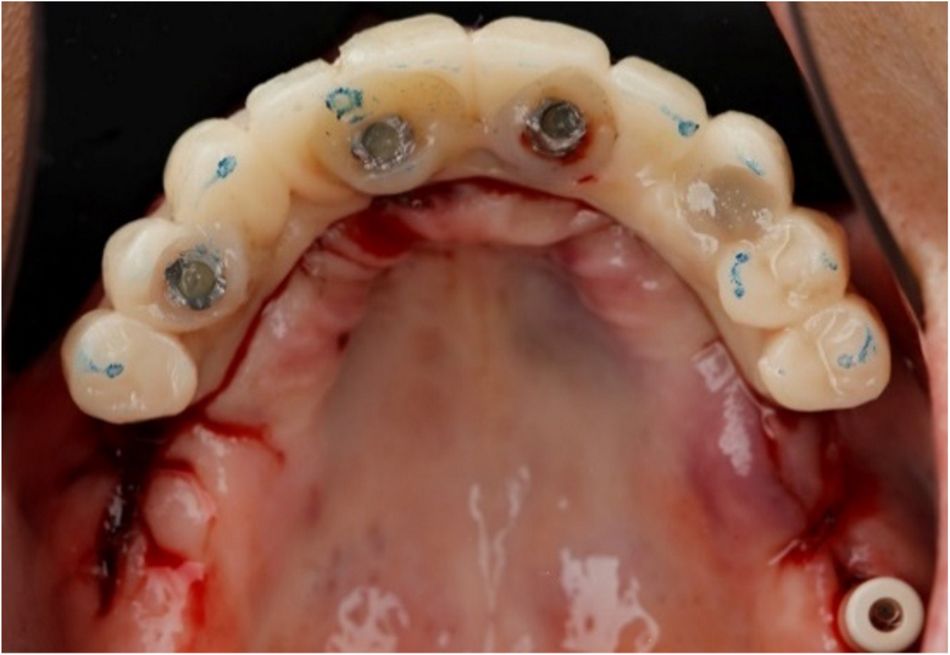
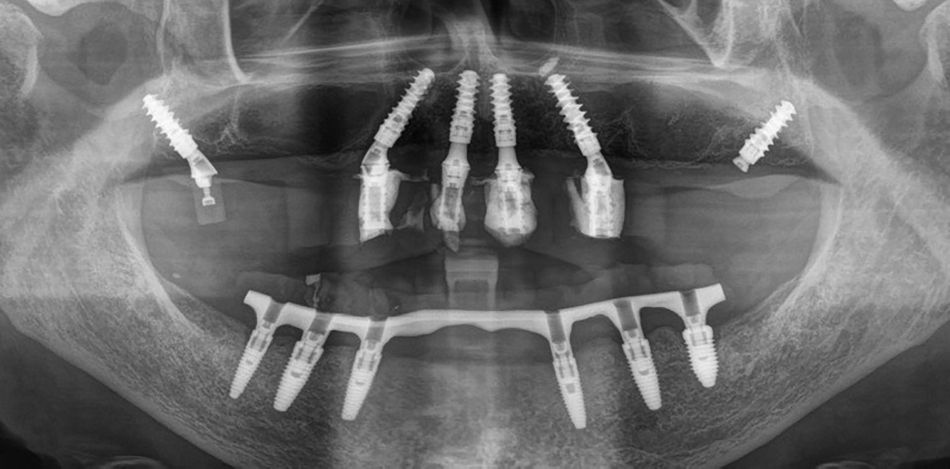
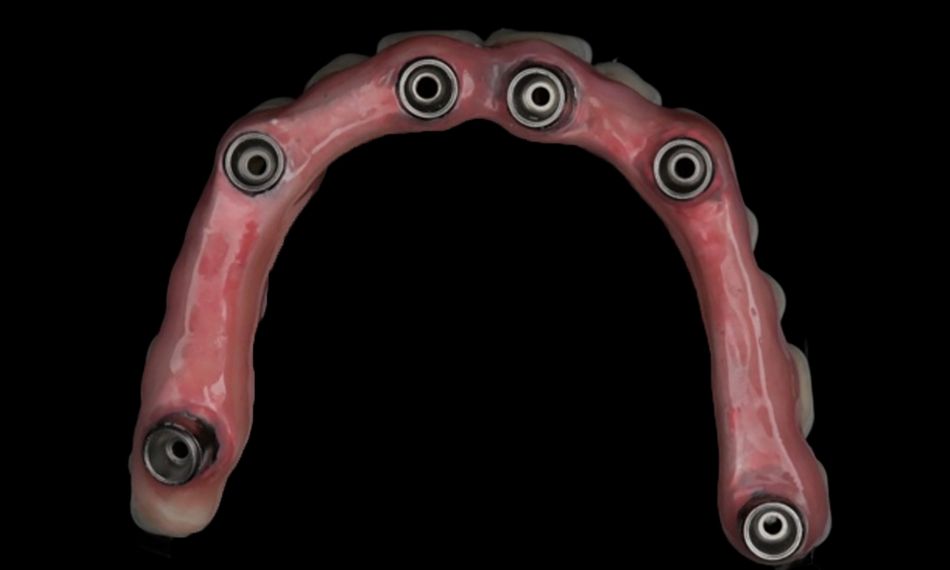
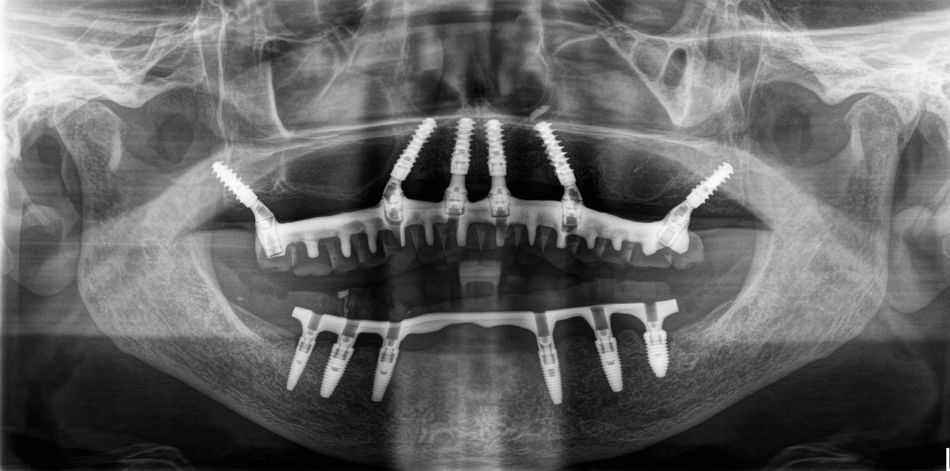
This case report focuses on the application of the Straumann® Pro Arch, which enables personalized treatment protocols for implant-borne fixed full-arch restorations. In this case, six BLX implants were placed in a fully edentulous patient with compromised oral function. The Straumann Pro Arch offers an efficient approach to full arch rehabilitation, combining the precision of guided surgery with the efficiency of immediate function. By integrating BLX implants into this treatment, clinicians can achieve stable and predictable outcomes, even in complex clinical situations.
The report outlines the clinical planning, surgical techniques, and prosthetic procedures involved in this case. It also highlights the rationale behind choosing the BLX implants, emphasizing how this approach can lead to predictable results in full arch restorations. Through this case, we aim to demonstrate the practical benefits of the Straumann® Pro Arch in delivering effective and lasting patient care.
Initial situation
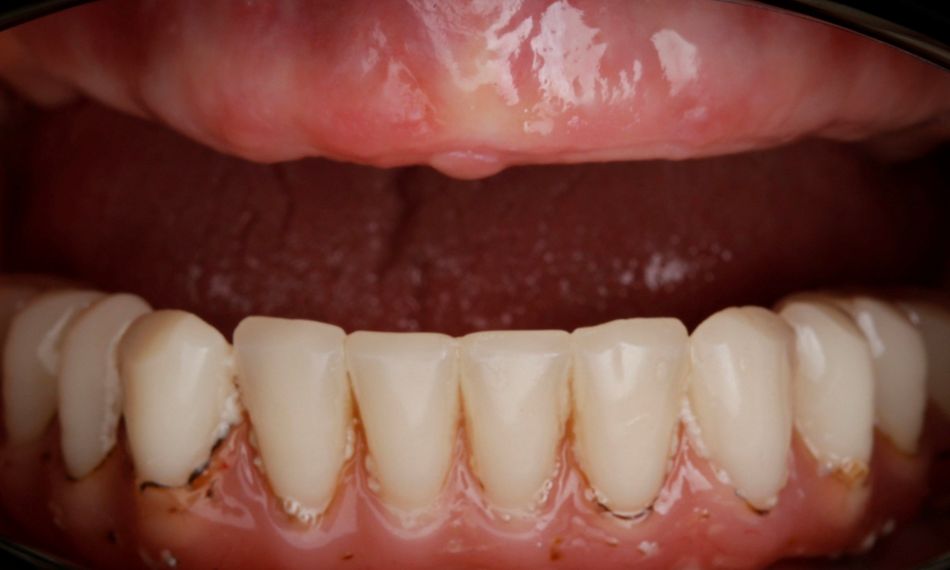
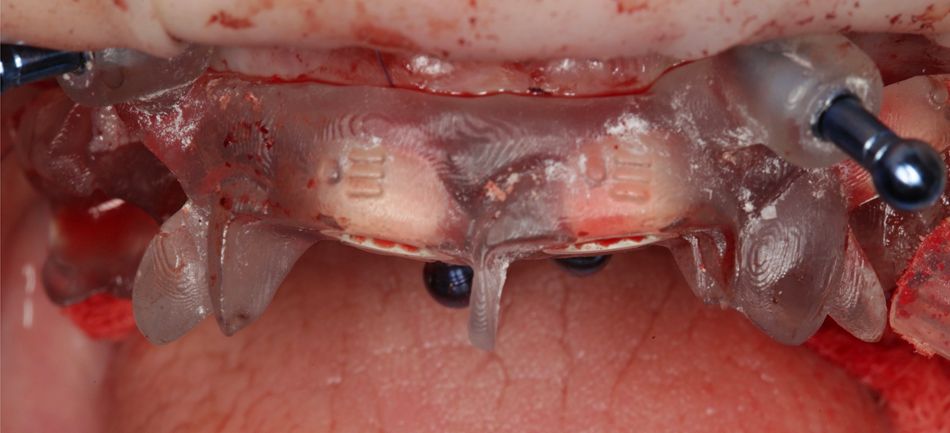
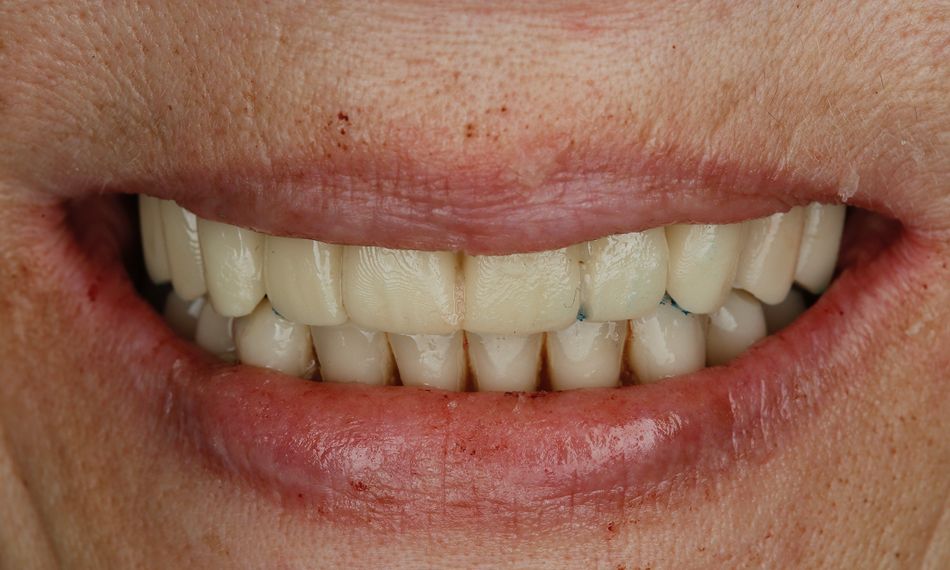
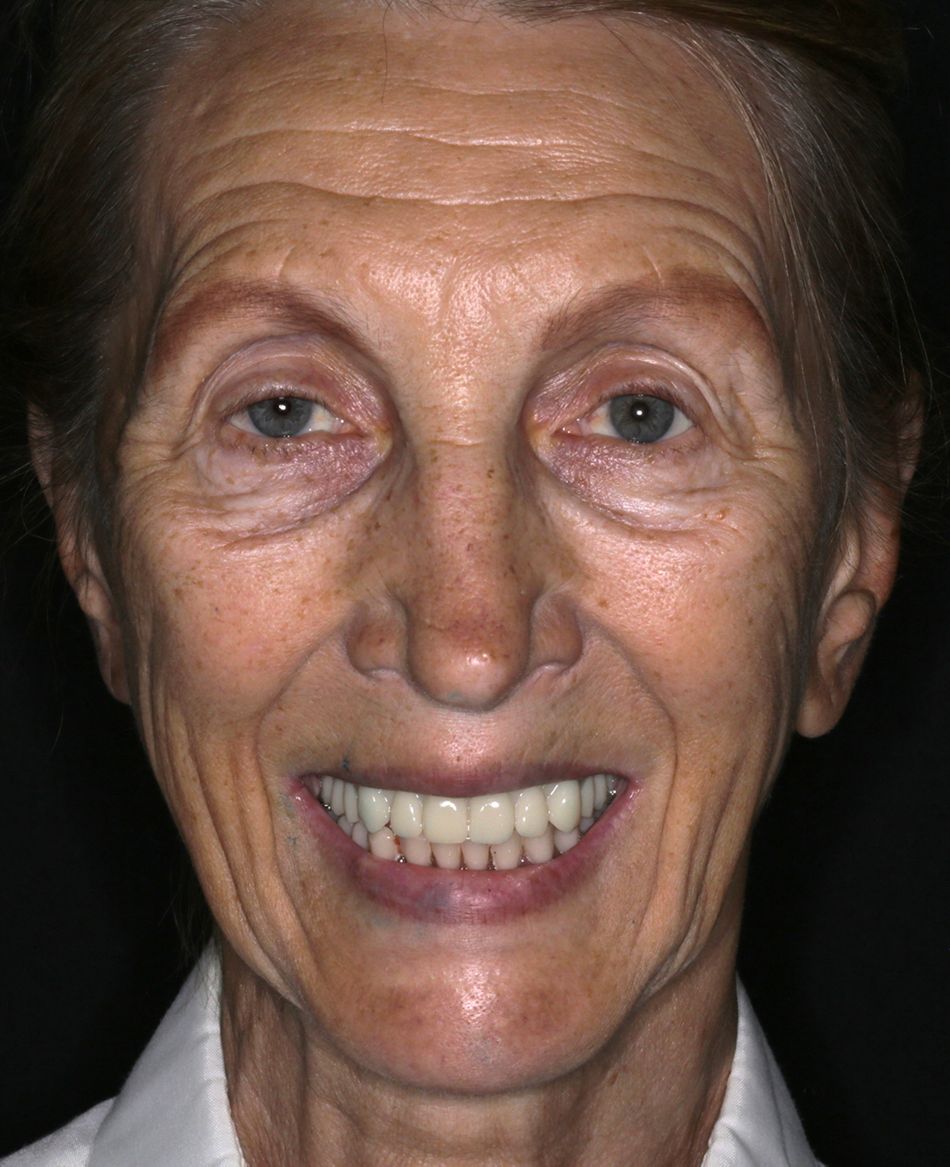
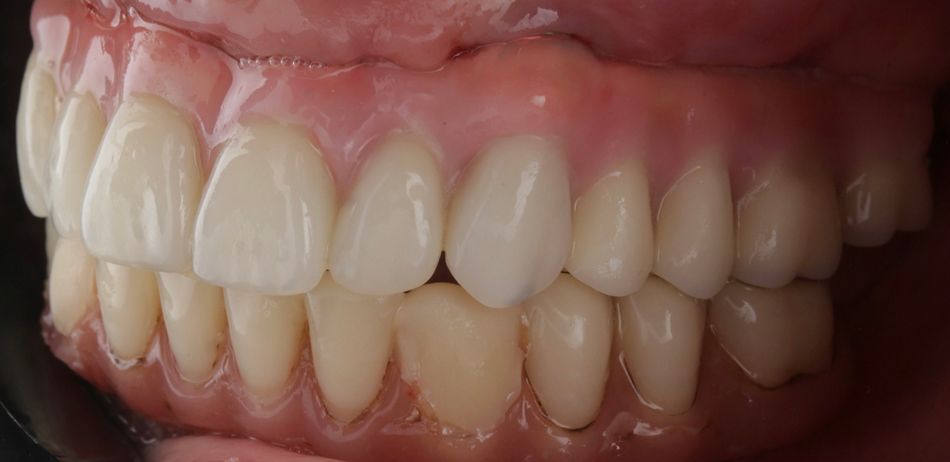
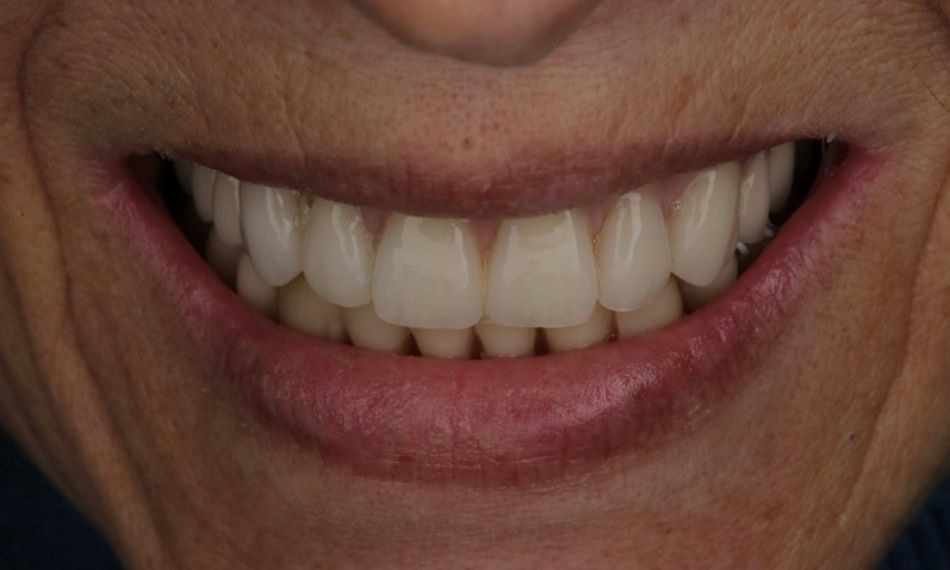
A 75-year-old female patient, classified as ASA I and a non-smoker, presented to our clinic with terminal dentition. She reported significant difficulty in chewing due to the instability of her upper denture and expressed a desire for a stable solution to rehabilitate her failing dentition and regain confidence in her ability to chew comfortably. Her primary request was to improve her current situation, enabling her to eat comfortably and confidently, thereby significantly enhancing her quality of life. During the extraoral examination, various parameters were evaluated. The patient demonstrated an adequate vertical dimension of occlusion and an appropriate incisal edge position. The position of her maxilla was found to be within normal ranges. The esthetics were acceptable. However, she had some difficulty with speech and, more significantly, with function, as the prosthesis was not stable (Figs. 1-3)
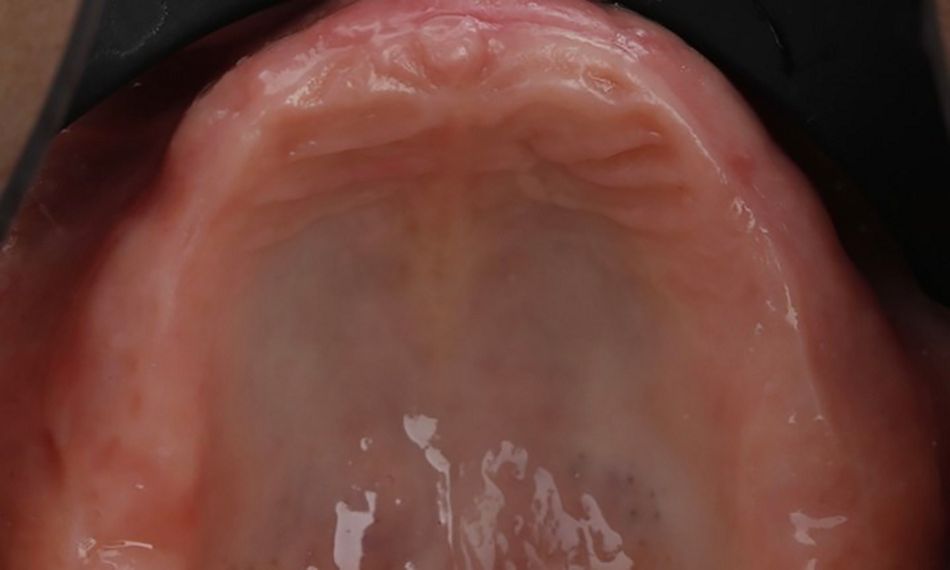
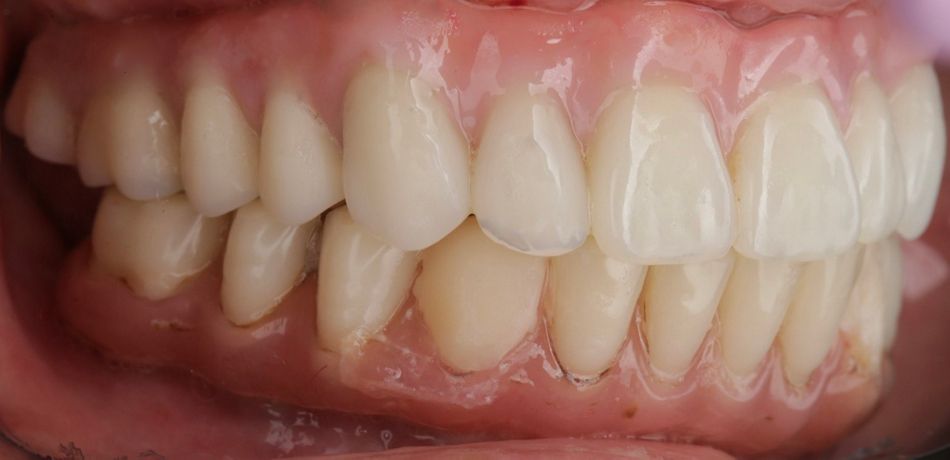
During the intraoral examination, the upper denture showed a lack of retention and stability. A midline discrepancy in relation to the facial midline was noted. Additionally, the patient presented with imbalanced occlusion when wearing the denture. After removing the upper denture, no intraoral pathology was detected (Figs. 4 & 5).